10 Key Questions About Process Validation
In other words, Process Validation in the food and beverage industry is the initiative of generating science-based data to prove and document the efficacy of a process in achieving its intended safety effect, i.e., its capability to effectively kill pathogenic microorganisms. Process Validation scientifically confirms that one food process, under one specific production configuration, allows delivery of a food product that is microbiologically safe.
A pathogenic microorganism, or pathogen, is usually defined as a microorganism that causes, or can cause, disease. These may be viruses, bacteria, parasites, or other types of microorganisms.
Many different pathogens can contaminate foods. They are called foodborne pathogens and can cause foodborne illness events. Depending on the foodborne pathogen involved, foodborne illness events can range from a small stomachache to more serious cases needing hospitalization, having long term effects on health, or causing death
Because each configuration is unique.
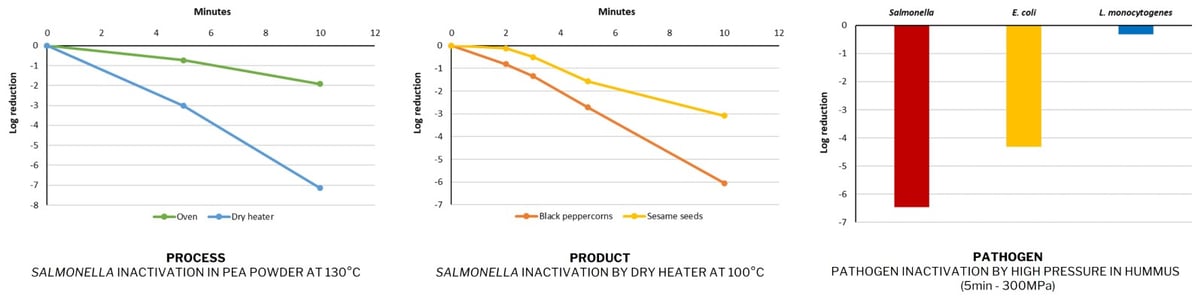
It is linked to what we call the “3P Rule”: Process / Pathogen / Product.
Each process has a different impact on the target pathogen, each pathogen reacts differently to a treatment, and each food product has intrinsic properties that have an impact on treatment efficacy. Below are examples from Novolyze's internal R&D illustrating the “3P Rule.”
The most important reason is to protect consumers by providing assurance that the food they consume is safe and of the highest quality.
Nevertheless, Process Validation is also important for the food and beverage industry as it is a key milestone for any food manufacturer who wants to be compliant with regulations.
Indeed, by reducing the risk of producing unsafe food, it also reduces the risk of costly recalls that can be associated with – in addition to foodborne outbreaks – economic losses, degradation of brand image, and failure of customer trust if a health scandal breaks out.
Yes, in most cases Process Validation is mandatory for food products. Many countries and organizations, such as the U.S. Food and Drug Administration and the European Commission, have regulations and guidelines that require companies to perform Process Validation studies to ensure that food products are safe.
For instance, the U.S. Food Safety Modernization Act (FSMA), enacted in 2012, put a specific focus on shifting from managing food safety failures to preventing foodborne illnesses, especially by promoting preventive methods such as Process Validation.
Without Process Validation, companies may face fines or penalties for failing to meet food safety standards.
Process Validation should be performed:
-
When a new process is introduced,
-
When a process is modified,
-
When a process is not meeting required standards,
-
When new elements are introduced to a food production process, such as novel applications, ingredients, and product reformulations that may introduce new risk areas to be checked,
-
Periodically, to ensure that the process is consistently producing safe and high-quality food products.
-
The Food Safety Modernisation Act (FSMA) mandates a regular review of Process Validations every three years. This review may also prompt a re-analysis of safety requirements, which in turn can lead to a new Process Validation procedure that replaces the initial validation.
-
A Process Validation team commonly includes (non-exhaustive):
-
A project manager, to coordinate the study and actions of other team members,
-
A food safety expert / food process authority, to ensure the study consistency,
-
A quality manager and engineers, to support the project manager and food safety expert for study preparation and execution,
-
A Process engineers, to anticipate process-linked pain points while designing the validation methodology,
-
A Process operators, to ensure good process functioning during the in-plant trials,
-
A laboratory team, to analyze the validation samples.
It is recommended to have a third-party expert leading the validation work, as well as a third-party lab for sample analysis. This is a better guarantee of results and validation study robustness, mitigating the risk of having any conflict of interest.
The methods that can be used to perform Process Validation include:
-
Literature review, to find evidence in the scientific literature that your process configuration is safe,
-
Mathematical modeling, to predict the behavior of your process and its expected lethality,
-
Benchtop trials, to generate science-based evidences of process lethality at lab or pilot scale,
-
And in-plant trials, to generate science-based evidences of process lethality directly from the process itself.
The advantages and drawbacks of each method are mainly:
-
Literature review
-
(+) Can be quickly implemented and less expensive than other options.
-
(-) Not always possible to find literature data accurate enough to document your specific process configuration.
-
-
Mathematical modeling
-
(+) Predicts potential risks and allows to anticipate them to keep the product safe.
-
(-) Costly, time-consuming, and needs a huge set of data to feed the model.
-
-
Benchtop trials
-
(+) Generates scientific evidence of a process efficiency at lab or pilot-scale, which is less costly and sometimes easier than working on the industrial-scale process.
-
(-) Scalability of the data generated on pathogen knowledge can be challenged (process reproducibility and variability).
-
-
In-plant validation with surrogate microorganisms
-
(+) Generates real-life scientific data directly from the target process, taking into account its variability.
-
(-) Requires confirming the suitability of the surrogate to mimic the pathogen of interest, through literature review or lab study.
-
Pathogens cannot be used to validate an in-plant process. Bringing Salmonella, Listeria, or any other pathogen into the plant environment for a validation study is non-sense as it would put the cat among the pigeons!
Surrogate microorganisms are non-pathogenic microbes that, when exposed to a specific stress (e.g., heat), behave similar to pathogens, having the same or slightly higher resistance to treatment than the pathogen they mimic. Their safety and suitability are properly characterized in lab before to use them for in-plant validation. They are not harmful and cannot endanger human beings.
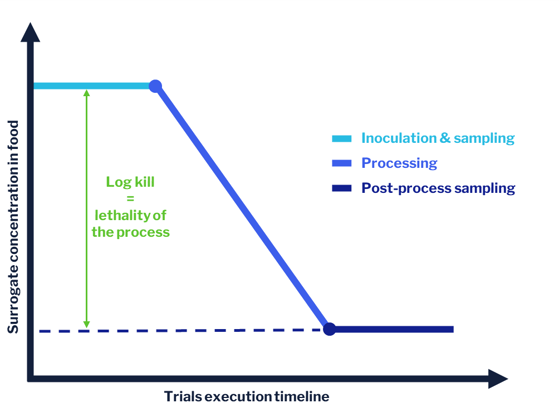
In-plant validation using surrogate microorganisms hinges on two main steps:
-
Mixing the food product with the surrogate – we talk about “inoculation.”
-
Exposing the inoculated food product to the process/treatment to be validated.
By taking food samples before and after the process step, we can estimate the levels of surrogate microorganism in food before and after processing, and the difference tells us what impact the process has on microbial viability.