Pitfalls of Finished Product Testing and Recalls
Did you know that the two most common causes of major global food safety incidents and recalls are undeclared allergens and cross-contaminations?
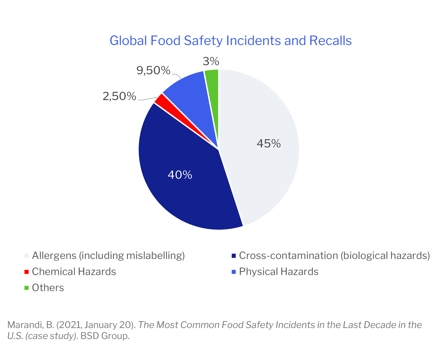
Recall and withdrawal events pose a significant risk for food manufacturing and distribution companies; they have the possibility of creating a public health crisis. Studies performed by the Food Marketing Institute and Grocery Manufacturers Association revealed that a food recall could cost a company as much as $10 million (direct cost). In addition, companies may also face indirect costs from brand and reputational damage, resulting in a loss of confidence by consumers in their products. Therefore, food manufacturers must adopt risk-mitigating strategies that are proactive rather than reactive.
Finished product testing is one of the food safety strategies companies employ at the end of the production process as a control measure to prevent or reduce food safety hazards. Theoretically, finished product testing, when executed as a control measure by quality assurance professionals, can verify the effectiveness of a food safety system and may be valuable in certain circumstances; for example, in traditional lot batch testing with a hold/release program. However, the results derived from finished testing products provide minimal information on the real-time safety status of the food due to statistical limitations associated with this test method which assumes that contamination is homogeneously (evenly) distributed throughout the tested product batch. 
The statistical futility of this assumption becomes apparent when product contaminants are present at low levels and heterogeneously (unevenly) distributed. The sensitivity of finished product testing is limited; it cannot ensure the conformance of a product lot to safety requirements, creating a false sense of food safety.
Moreover, the costs associated with product testing are often high because while the finished product testing is ongoing, related batch products are put on hold and released at test completion (this depletes company resources).
The shortcomings observed with finished product testing should prompt food and beverage manufacturers to deploy a better and more robust approach in their food safety management system. Control and assurance practices that strategically incorporate superior preventive controls (environmental monitoring programs, good hygiene practices) and verification processes (validation, verification, and documentation) are more effective than reliance on finished product testing.
Keys to Recall Prevention
Process Validation
An efficient food management system should build its foundation on sound, scientific evidence that can demonstrate the control of potential hazards in real-time. Food companies' dependence on finished product testing as a preventive measure to establish food safety before their release down the supply chain may be assumptive because, in most instances, these measures are not adequately validated.
A prudent approach will be required to achieve optimal food safety and involves performing careful hazard analysis and process control validation by qualified professionals to determine and understand the risk associated with the food production process. The food safety system is deemed valid through concrete scientific evidence generated by process validation.
Validation is considered an ongoing part of the system established initially but may be subject to revision in the future if changes occur in certain aspects of the operations, food safety objectives, ingredients, processes, etc.  Food companies can leverage the insights gained from conducting validations to optimize control measures associated with the production process and adjust their food safety plan to achieve optimal processing parameters.
Food companies can leverage the insights gained from conducting validations to optimize control measures associated with the production process and adjust their food safety plan to achieve optimal processing parameters.
The three strategic approaches typicall used in the validation process:
1. Prospective validation
-
Performed before a new product undergoes distribution or changes to its manufacturing process that may affect its food safety profile
-
Used in validating novel preservation methods, sterilization, and aseptic processes
2. Concurrent validation
-
Performed concurrently with manufacturing process
-
There is usually significant level in confdence on validation outcome because of data generated and reviewed under a full prospective validation of the process or product
3. Retrospective validation
-
Validates a process for a product that is already in distribution. Typically relies upon accumulated production, testing, and control data.
Verification
Once a process demonstrates to be under control through validation, trained personnel can initiate verification activities to ensure the consistent and effective execution of the preventive controls. When verifying preventive control measures, the assurance activities should include actions that confirm the efficacy of all elements of a food safety plan. It should consistently show that the preventive controls are sufficient for their intended purpose and can control potential hazards.
Examples of verification activities include:
-
Review of the food safety plan and its records
- Review of deviation analyses and product dispositions,
- Ensuring that proper change control procedures are in place and adhered to
- Product sampling
Environmental Monitoring Program
The practical application of an environmental monitoring program significantly defines the ability of a food manufacturer to achieve microbial process control of its environment hence its finished product. A solid prerequisite program should incorporate environmental monitoring to measure the imminent risk posed to the food processing environment and assess the effectiveness of hurdle steps established to prevent the entry of microorganisms into the manufacturing facility. Typically, this entails individual and concurrent sampling of process control, indicator sites, and verification sites.

Sample sites for environmental monitoring programs are selected based upon risk as identified in HACCP, zoning, and hygienic design review, with the primary focus on the following areas:
-
Challenging to clean areas around the equipment that could be microbial harborage sites
-
High-traffic areas
-
Interfaces where movement occurs between hygiene zones
-
Interfaces between areas where raw, highly contaminated materials handling occurs and areas where processed materials treating occurs after the application of a microbiocidal process (such as cooking)
-
Interfaces between wet- and dry-clean areas
-
Areas from where pathogens could be transferred into sensitive areas with the exposed product or raw materials through the movement of people, equipment, and materials
-
Areas where microorganisms could enter the facility from outside the factory or from higher risk areas within the factory
The results obtained through the environmental monitoring program provide valuable insights into the level of sanitary control achieved in the facility and aid in identifying cross-contamination risks and preventive control failures.
Microbiological process control is a three-step process:
1. Eliminate the resident organisms of concern from the processing environment
2. Control movement by managing the vectors and pathways
3. Utilize process control methodology to measure and predict loss of control
The concept of complete microbiological process control uses environmental monitoring to measure the level of control.
Other Preventive Measures to Consider
Food and beverage manufacturers should adopt a safety culture and have food safety teams responsible for preparing, implementing, and documenting an approved food safety plan. Companies need diligent preventative measures to assure quality throughout the manufacturing process. Lack of process, sanitary, and allergen controls are the leading contamination causes resulting in a recall event.
Utilization of Quality Management Software (QMS) solution provides food companies data in real-time to gain insights into their quality program (potential product non-conformance, verify product specification, traceability). Quality managers can calibrate or modify critical control points (CCP's) throughout the manufacturing process to prevent or eliminate hazards based on real-time data derived from QMS, making decision-making faster. Built-in automatic alerts within the system assist in identifying exposure before further contamination can occur.
QMS solutions make tracking allergens in the supply chain and manufacturing processes for food and beverage manufacturers less time-consuming. In addition, it makes it easier to avoid allergen and cross-contamination and adhere to regulatory, non-regulatory, and customer mandates (lot tracking, stringent sanitation practices, and raw material segregation).
 While finished product testing may be helpful for verification activities in a food safety system, its ability to proactively provide evidence on the safety of food in real-time is lacking. A more prudent approach by food and beverage companies would be to integrate risk-mitigation methods, including validation and verification activities and environmental monitoring programs capable of searching for and destroying potential hazards. This strategy reinforces their continuous improvement efforts and makes it easier to prevent food recall events and foodborne illness, eliminating the potential of a public health crisis.
While finished product testing may be helpful for verification activities in a food safety system, its ability to proactively provide evidence on the safety of food in real-time is lacking. A more prudent approach by food and beverage companies would be to integrate risk-mitigation methods, including validation and verification activities and environmental monitoring programs capable of searching for and destroying potential hazards. This strategy reinforces their continuous improvement efforts and makes it easier to prevent food recall events and foodborne illness, eliminating the potential of a public health crisis.
Learn more about our solutions to streamline efficiencies in food safety, quality and compliance.





