Regulatory & Recall Round Up - January 2022
In this month's Regulatory & Recall Round Up, learn about the Egg Regulatory Program Standards, presented by the FDA and the National Egg Regulatory Officials on January 14. You will also find a recap of 2021 US outbreaks & recalls.
In compliance with FSMA, FDA lays out new standards for Eggs
The Food Safety Modernization Act (FSMA) mandates that the U.S. Food and Drug Administration (FDA) establish a National Integrated Food Safety System (IFSS) which includes partnerships across the expanse of federal, state and local agencies. As part of this FSMA requirement on January 14, 2022, the FDA and the National Egg Regulatory Officials (NERO) presented the Egg Regulatory Program Standards (ERPS) , a program which provides state regulators of egg and egg products with best practices for the implementation of their egg and egg-product regulatory programs.

Regulation of eggs and egg products is handled by FDA (FDA Egg Guidance Documents), USDA, as well as state regulators. At the federal level, FDA regulates shell eggs while USDA regulates egg products. Many states conduct egg inspections per the requirements of state laws and regulations and/or the Federal Food, Drug, and Cosmetic Act, this includes contracted out egg inspections by FDA to the states. ERPS is intended to encourage uniformity in this complex regulatory landscape and to provide a model system to address the potential of food-borne illnesses.
A classic evaluation and conformance program, ERPS conformance will drive a process of continuous improvement and collaboration between the FDA, regulatory agencies and producers of eggs and egg products. ERPS provides a set of regulatory best practices termed the Program Standards.
The Program Standards are comprised of ten standards that establish requirements for the core components of a regulatory program related to food safety and quality issues from egg and egg products. The Program Standards are comprised of: regulatory foundation, staff training, inspections, quality assurance, egg and egg product-related incident response, enforcement, industry outreach, resource management, program assessment and laboratory support.
The ten standards specifically detailed in ERPS consists of the following, that will establish requirements for a model state regulatory program:
-
Regulatory Foundation – evaluation of the state program’s regulatory authority;
-
Staff Training – written training plans and continuing education;
-
Inspection – written safety inspection and sampling procedures;
-
Quality Assurance – written procedures to audit the effectiveness of inspection and sampling;
-
Egg and Egg Product-related Incident Response – written procedures to respond to contamination or adulteration of egg or egg product incidents;
-
Enforcement – a documented enforcement program;
-
Industry Outreach – outreach and communication to stakeholders;
-
Resource Management – assessment of the resource needs of the program;
-
Program Assessment – periodic reassessment of the regulatory program against the criteria established by ERPS; and
-
Laboratory Support – adequate access to labs to support the program.
2021 Outbreak and Recall update in the US
This information has been compiled by Laure Pujol, PhD, Associate Research Fellow at Novolyze.
In 2021, 18 outbreaks have been reported causing a total of 1327 illnesses, 325 hospitalizations and 7 deaths. The majority of illnesses and hospitalizations are due to Salmonella contamination. However, the majority of deaths are related to Listeria monocytogenes contaminations. (https://www.cdc.gov/foodsafety/outbreaks/multistate-outbreaks/outbreaks-list.html )
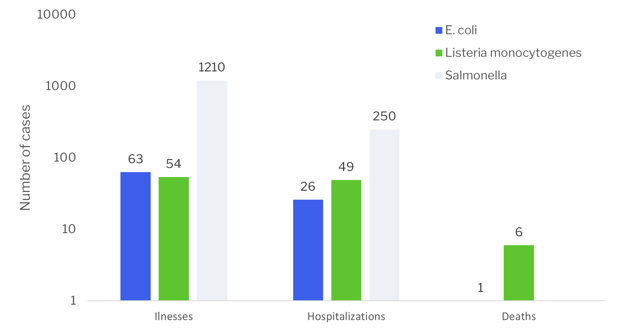
In 2021, a total of 233 recalls have been declared. (https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts)
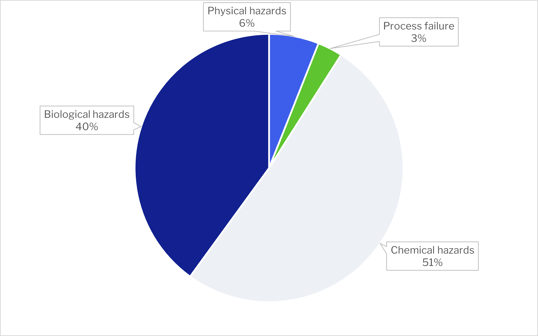
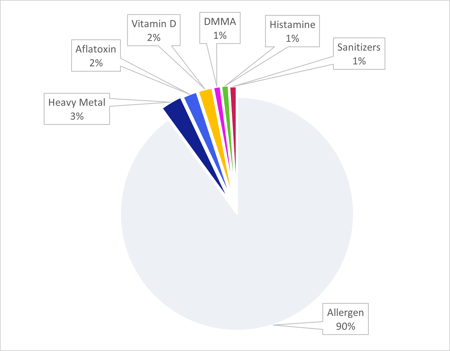
Over half of the recalls were due to chemical hazards. The main cause of chemical hazard recalls was by far allergens, representing 90% of recalls.
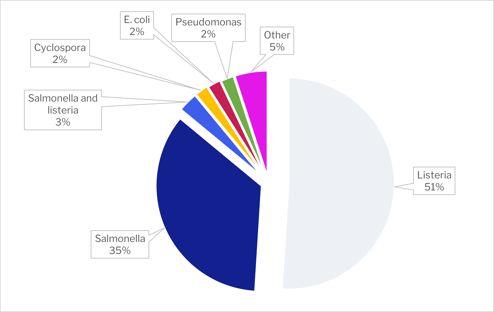
Biological hazards represented 40% of the recalls in 2021. Listeria was the main cause with 51%, followed by Salmonella (35%).
Don't miss our upcoming Regulatory & Recall Round Up. Subscribe to our blog.




